HISTORY OF CHLORINE DIOXIDE
Sir Humphrey Davy, a British chemist, discovers chlorine dioxide (CD), a result of potassium chlorate reacting with sulfuric acid. Although he fully understood the chemistry, he most likely did not appreciate the consequences: CD will prove to be more effective at killing more viruses, bacteria and fungi than any comparable disinfectant, such as chlorine bleach.
1930s
Because of the disinfecting power of CD, use begins to grow. A major benefit of CD is that, as a true gas, it expands uniformly to fill the space it’s disinfecting. Due to concerns about the logistics of safely transporting the gas, industries wishing to use it decide to simply make it themselves in large quantities and activate it on site.
1944
To mitigate taste and odor problems, CD is introduces into a water treatment plant at Niagara Falls, N.Y. Other municipalities soon do the same. The water not only tastes better and has no unpleasant odor – it’s also safer to drink, thanks to CD’s strong disinfecting properties.
1950s
Brussels, Belgium, changes from chlorine to CD to disinfect drinking water in 1956. The 1950s see widespread use of CD in water treatment plants and swimming pools in the U.S. A new discovery is made: CD destroys biofilm, the algal slime that collects in cooling towers, among other places and harbors harmful bacteria. Chlorine bleach by contrast cannot kill biofilm.
1967
The Environmental Protection Agency (EPA) registers an aqueous form of CD for use as a sanitizer and disinfectant. Even though it aggressively attacks pathogens it is extremely safe and CD is eventually used to commercially sanitize fruit.
1970s to early 1980s
The EPA begins recommending using CD instead of chlorine bleach to treat water because CD does not produce any harmful byproducts. Although chlorine and chlorine dioxide share a common name, they are fundamentally different chemicals with distinctly different chemical structures. This means they react differently when mixed with other compounds. Chlorine bleach is formed by adding chlorine gas to salt water. However, when chlorine bleach reacts with naturally occurring organic and inorganic matter in water streams, it produces THMs (trihalomethanes). THMs have been linked to cancer. Because of its superior efficacy and how safe is its, use of CD continues to grow.
1988
The EPA registers CD as a sterilizer. This means CD is both safe and effective to use in hospitals, healthcare facilities, and laboratories.
2001
CD, in both liquid and gas forms, becomes the number one substance used to decontaminate buildings where the anthrax attacks occurred. It’s success rate is based on how well is works against very small anthrax spores, how quickly and easily it is deploy. CD completely destroys anthrax without harming buildings.
2005
CD is deployed to eradicate mold infestations in homes damages by the flood waters from Hurricane Katrina. After a 12-hour treatment, a New Orleans restaurant is able to banish all mold inside without rebuilding.
2012 (January)
ProKure revolutionizes the way CD is deployed. For the first time, because of ProKure’s patented technology, CD can be created at any time, and anywhere there’s water. It can now be safely transported in dry pouches and made into a liquid disinfectant and deodorizer on site and on demand. In essence, the ProKure product line has made it possible for industries and companies of all sizes (not just a select few) to quickly and easily unleash the amazing power of chlorine dioxide.
WHY?
CD is now two hundred years old. Chlorine Dioxide is used as a disinfectant against the Ebola virus. It begins to kill pathogens in a matter of seconds, whereas other commonly used, more traditional disinfectants take minutes. The rapid speed in which ProKure V kills pathogens makes it a product of choice for helping contain infectious-disease outbreaks and keeping public facilities cleaners and safer for everyone.
Why chlorine dioxide?
The active ingredient that kills most lethal, hard-to-kill and resistant pathogens, such as Ebola, MRSA, anthrax, norovirus, pseuodomonas aeruginosa and C. diff is chlorine dioxide.
Better at killing pathogens
Chlorine dioxide and bleach (NaOCl, or HOCl) both kill organisms by denaturing, or altering, the proteins within them. They do this through oxidation of the protein building blocks known as amino acids. But whereas bleach reacts with a wide range of amino acids, chlorine dioxide reacts with just a select few. This means it takes far less chlorine dioxide to kill organisms off than it does bleach.
Chlorine dioxide has another critical advantage over bleach: it can penetrate biofilm to kill pathogens. Biofilms are slimy layers of biological materials that accumulate wherever water is found; microbessecrete protective coatings that form a filmy later. Biofilm is high in yuck factor—think slimy pipes and sticky dental plaque. Many bacteria thrive in biofilms.
Bleach reacts with biofilm and is consumed by it before it reaches the pathogen. Chlorine dioxide does not react with biofilm, nor is it consumed by it, instead it easily penetrates the protective coating to kill the pathogens. Without living pathogens, the biofilm is also destroyed.
Super-stalker of superbugs
Chlorine dioxide physically kills cells through oxidation of their outer layers, literally burning them apart. This basically leaves no possibility for the kind of mutation that results in the rise of “superbugs”—such as the strains of bacteria that become resistant to disinfectants and antibiotics. For the few bacteria that do have a built-in defense mechanism against oxidation, the protective molecules are few, and chlorine dioxide can easily overcome them. It just takes a little bit more of the solution, or a higher concentration of it.
Chlorine dioxide is basically a take-no-prisoners solution to superbugs. It kills off the organisms completely, including the small number that may have built-in resistance to other chemicals.
Better than bleach
Chlorine dioxide is FASTER.
The treatment time is seconds to minutes, vs. minutes to hours for bleach. Thus, the pathogen is eradicated more quickly. Plus, chlorine dioxide saturates evenly and completely, on both hard and soft surfaces, resulting in a time-saving application.
Chlorine dioxide is STRONGER.
The broad spectrum of pathogens that chlorine dioxide eradicates includes aerobic, non-aerobic, gram-positive and gram-negative bacteria, spores, viruses and fungi. Bleach is ineffective above pH7 and cannot be safely used above 40° C / 104°F. Chlorine dioxide is not pH-dependent and can still do its job at higher temperatures. Chlorine dioxide removes the clusters of bacterial cells known as biofilm; bleach does not. Chlorine dioxide requires much lower concentrations to work—50 to 1,000 ppm versus 5,000 to 10,000 ppm for bleach.
Chlorine dioxide is SAFER.
Unlike bleach, chlorine dioxide does not form harmful or carcinogenic chemicals when it’s used as a disinfectant. Bleach reacts with more compounds, including toxic compounds, than chlorine dioxide does. Because bleach reacts with more compounds, more of it is needed to disinfect. Additionally, because bleach becomes bound with organic molecules, it cannot disinfect organic materials such as spills from bodily fluids.
Chlorine dioxide, by contrast, can effectively disinfect organic substances such as these. Chlorine dioxide is also preferable to antimicrobial cleaners, as the antimicrobial compounds can accumulate in humans and contribute to the development of resistant strains of pathogens. The lack of byproducts with chlorine dioxide eliminates the need for neutralization before emptying the used solution into a drain. In comparison, bleach solutions do need to be neutralized first. Bleach forms chlorinated phenols, which deplete the ozone layer. Chlorine dioxide does not; it will not deplete the ozone layer. Chlorine dioxide is also less corrosive than bleach, and new production methods for chlorine dioxide mean that it does not corrode stainless steel, a common surface in hospitals and other healthcare facilities.
To purchase this product for disinfection uses go to: ProKure
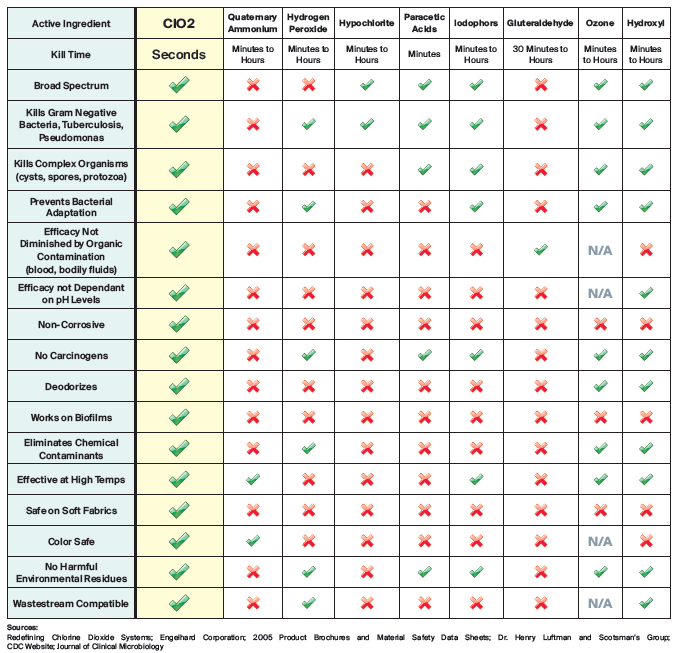
Disinfectant Comparison
There are thousands of disinfectants. Trying to find the best one is time-consuming and difficult.Below is a chart that allows you to see why we believe that ProKure V is the ultimate disinfectant. The active ingredient in ProKure, chlorine dioxide, provides more benefits and advantages than any active ingredient found in other disinfectants.